Exploding photographers, disappearing clothes, and the development of film
posted Tuesday, May 1, 2012 at 1:49 PM EST
 It’s been a while since I wrote a history article and two or three people seemed to like them. Since I’m one of those two or three people, and it’s my blog, I figured it was about time to do another one. I’ve pretty much covered the development of early cameras and lenses so it’s time to consider the way we recorded those images so other people could see them. No, I’m not talking about Facebook. I’m talking about film. Actually, I’m talking about even before film, mostly, but I really wanted to work that ‘development of film’ bit into the title. Pretty great, isn’t it? OK, maybe not.
It’s been a while since I wrote a history article and two or three people seemed to like them. Since I’m one of those two or three people, and it’s my blog, I figured it was about time to do another one. I’ve pretty much covered the development of early cameras and lenses so it’s time to consider the way we recorded those images so other people could see them. No, I’m not talking about Facebook. I’m talking about film. Actually, I’m talking about even before film, mostly, but I really wanted to work that ‘development of film’ bit into the title. Pretty great, isn’t it? OK, maybe not.
The First Images
The very first cameras, of course, were Daguerrotypes and the images they made were positives on silver plates coated with Iodine and developed using fumes from Mercury. You can probably already tell this had a few drawbacks. Positive images can’t be reproduced so one picture was one picture — if you wanted a copy for Aunt Bessie you had to take another picture. Silver is silver, so each picture was rather pricey (up to a month’s pay for a working man). I guess inhaling mercury fumes in the darkroom all day didn’t exactly lead to a lot of healthy old photographers walking around either.
Not long after that, the albumen process was developed. This let photographers make negative images on glass plates coated with albumen. Glass is a lot cheaper than silver, which helped make photographs affordable. Since the images were negatives you could make as many prints as you might like from a single photograph, so things like picture books came into being. Images on glass could be projected in ‘magic lanterns’ so risque images of ladies’ ankles and such could be projected at the gentlemen’s clubs of the day. So the albumen process made it possible for photographers to achieve the same goals they have today: getting published in book form and getting pretty girls to pose partially undressed.
Albumen had it’s drawbacks, though. The process was difficult and time consuming, requiring the plates to be prepared fresh just before each photographic shot. Carrying around a few hundred glass plates got rather heavy, and glass breaks. And the major source of albumen, in case you don’t know, is from egg whites. Photography became so popular that it actually led to egg shortages. As many as 1,000,000 eggs a year were used for photography in England alone.
Cellulose, Nitrocellulose, and Collodion
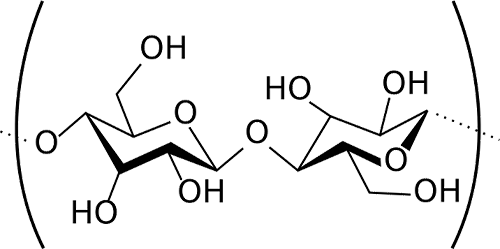
Oddly enough, the same year that Daguerre introduced the camera (1838) another Frenchman, Anselme Payen, discovered that a chemical called cellulose was the major structural component of most plants. Everything from cotton to wood was largely cellulose, in slightly different variations. Other chemists quickly found out that cellulose was a huge molecule, but made up of a small molecule (glucose) linked over-and-over into long chains.
|
Chemical diagram of two glucose molecules linked together, like they are in cellulose. A single cellulose molecule is made up of from 50 to many hundred glucose molecules. Image courtesy Wikimedia Commons. |
I promise not to go all chemical formula on you in this article, but it’s easier to show you a picture. The hexagon in the middle of each glucose molecule is a ring of carbon atoms, and you can see there are various atoms (Oxygen and Hydrogen) sticking out from the ring. The reason chemists were all pumped about this was if you made a chemical change to cellulose, you’d be making that change to hundreds of atoms that were linked together. Cellulose is available in everything from wood to cotton, so if you made some useful new chemical from it, you’d be able to get your starting supplies very cheaply. That’s always a good thing.
Schönbein and Exploding Cotton
Christian Friedrich Schönbein was a Professor of Chemistry at the University of Basel. He was also a complete chemistry geek. Working in the lab at the University all day wasn’t enough for Christian, he liked to do some experimenting at home after dinner and on the weekends. Christian’s wife had gotten pretty tired of this and forbidden him to do any more chemistry experiments at the house. But wives will be wives and go visit their sisters, and men will be men and use such opportunities to do what they are forbidden to do.
Christian decided to take advantage of his afternoon home alone to play with Nitric Acid and some other fun things. Of course, given the karma debt this caused, he spilled the acid on the kitchen floor and grabbed his wife’s cotton apron to mop it up with. He hung the apron over the stove to dry, no doubt checking his watch to see how much time he had before the little lady got home. He needn’t have bothered: the apron exploded rather violently, blowing the windows out of the kitchen and causing various other damage.
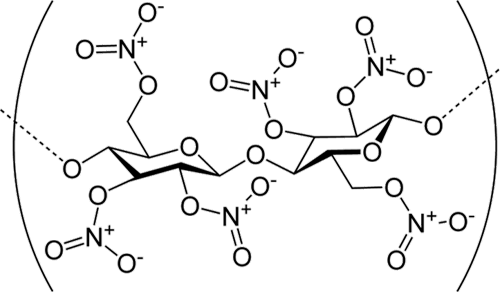
History didn’t record the discussion Christian and his wife had when she got home. But it does record that Christian had accidentally discovered guncotton. The Nitric Acid had converted all of the Oxygen-Hydrogen (OH) side chains to Nitrate side chains--the cellulose was now nitrocellulose (aka guncotton), which just loves to explode. I can assure you this is so--I first read about guncotton in college and my geek friends and I snuck into the Organic Chemistry lab to make some. In case you ever wanted to know, chemistry lab windows cost $145.55 each and if you blow some out you have to pay for them before you can graduate. (If you feel the need to repeat my actions, there’s a link in the references that tells you how to make your own guncotton. But remember, this is a first step to becoming a Darwin Award winner.)
|
Nitrocellulose, aka guncotton. Image courtesy Wikimedia Commons. |
Christian, being German, immediately realized that there would be big money selling his guncotton to the military: it was more powerful than gunpowder and didn’t leave huge clouds of smoke like gunpowder did. He immediately attracted some venture capital and built factories to manufacture guncotton. Unfortunately for Christian and his investors, one by one all of the factories exploded and they went bankrupt. Guncotton wasn’t all that safe to work with.
Collodion
Schönbein had been right, though. There was a huge market for guncotton if it could be made safe (safe explosive is kind of an oxymoron, but you know what I mean). Chemists all over the world began experimenting with cellulose and nitrates. They did make it safer, but they also learned other things. For instance, guncotton will dissolve in a solution of ether and alcohol, making a thick, syrupy liquid called collodion. When exposed to air it quickly dries to a plastic-like coating (you may have used it in products like “liquid bandage” or “compound W”).
 |
|
Frederick Scott Archer. Image courtesy Wikimedia Commons. |
Collodion was pretty cool and all, but other than instant bandages and making shiny coatings on things it didn’t appear to have a lot of uses. However, In 1850, Frederich Scott Archer found that collodion made a superb substitute for the albumen being used to coat glass plates for photography. Light sensitive chemicals dissolved in it easily, it coated plates smoothly, it cost less than albumen, and it didn’t spoil. (Albumen comes from eggs. You know what three day old eggs smell like?). Most importantly it let people go back to using eggs for their natural purpose--breakfast.
The importance of Archer’s discovery really can’t be exaggerated. It became the primary way photographs were made by the mid 1850s. The process was further improved by W. B. Bolton and B. J. Sayce in the 1860s, making it somewhat simpler and more consistent.
It wasn’t perfect, of course. The plates had to be made by the photographer within 10 minutes or so of the exposure. It was sensitive only to blue light, so warm colors appear dark and cool colors light, so a person dressed in bright red or yellow would appear to be wearing dark clothes. Because clouds and sky are both shades of blue, clouds rarely are seen in collodion process images. Oh, I almost forgot--collodion is nitrocellulose (guncotton) suspended in ether and alcohol, so the odd fire or explosion did occur when preparing photographic plates.
A Photographer Discovers Synthetic Cloth
Toward the end of the collodion era, a French photographer, Hilaire de Chardonnet, spilled some of his collodion. When he cleaned up the partially dried puddle he noticed that the collodion pulled away in long, sticky threads. They reminded him of the threads of silkworms which he had seen as a student.
 |
|
Hilaire de Chardonnet. Image courtesy Wikimedia Commons. |
Chardonnet tried forcing collodion through small holes drilled in a metal plate and found it made nice shiny threads that could be woven into cloth. He patented the process in 1885, showed his “Chardonnet Silk” at the Paris exposition of 1889 to great acclaim, and opened factories to produce his marvelous new material in 1891.
Chardonnet Silk was very popular, for about a year. But Chardonnet forgot what we just talked about: collodion is largely nitrocellulose and so was the cloth he made from it. In one known incident, a gentleman accidentally flicked a cigar ash on the dress of his dancing partner. Her entire gown disappeared in a blinding flash of light and heat, leaving them both with flashburns and the lady displaying more of her charms than she had intended.
Chardonnet was able to make changes to his chemical process. (The fact that his original factory burned down in another nitrocellulose fire probably made it easier to do so.) By that time, however, other chemists had tried his “push a thick sticky substance through small holes to create threads” idea with other chemicals. One company found that viscose, which was created from the cellulose of wood chips, would make artificial threads just like collodion would, but with the advantage of not exploding. Viscose could not only be forced through small holes to make threads, it could be forced through thin slits to make sheets. The American Viscose Corporation and the DuPont Fibersilk Company produced both the cloth (which we call Rayon) and the sheets (Cellophane). The latter company made a major fortune and is still around today as E. I. du Pont de Nemours and Company, commonly called DuPont.
Dry Plates and Early Film
Despite it’s drawbacks and complexity, wet plate photography had a long life by today’s standards, over 20 years. Photographers, then, as now, were always searching for a better way, though. In the early 1870s, Richard Leach Maddox began putting silver bromide in a layer of gelatin on glass plates, and letting it dry--the birth of dry plate photography. It wasn’t popular at first, but a few years later Charles Harper Bennet found that heating the plates made them more stable and more light sensitive. Then, as now, increased light sensitivity attracts photographers like moths to a candle. Within a year or two, dry plate photography had largely replaced the collodion process.
In 1879, George Eastman invented a coating machine that mass produced dry plates. Suddenly photography became something people could do without years of training. Now there was no need to spend several minutes preparing a plate before taking a single photograph, you just unwrapped a dry plate and popped it in the camera. Eastman then had the brilliant idea of coating his gelatin emulsion on rolls of paper instead of sheets of glass. Since it had a paper backing, this first film wasn’t transparent like more modern film. After exposure the gelatin film layer was stripped from the paper, coated with (guess what?) collodion, forming a transparent negative that could be used to make prints.
Eastman was rather shocked that professional photographers didn’t flock to his paper film, but professionals seemed very threatened by the idea that someone without lengthy training would be able to make photographs. Charles Lutwidge Dodgson (aka Lewis Carroll) was, in addition to being an author, one of the premier portrait photographers of the wet plate era. When he first saw the new dry plates, his only remark was “Here comes the rabble”. He stopped taking photographs entirely a year later.
 |
|
Alice Lidell photographed by Charles Dodgson. She was the Alice who inspired Alice in Wonderland. Image courtesy Wikimedia Commons. |
Eastman was a shrewd businessman and realized for every professional already taking pictures, there were dozens of nonprofessionals who wanted to. He marketed his invention to the masses, making small, self-contained cameras that people could use with virtually no training. They could even send him their film for development and printing. Eastman named his company Kodak because he liked the letter ‘K’, he felt the name could not be mispronounced, and it didn’t resemble any word used in photography at that time. He wanted everyone to know his company was completely different from the photography suppliers of the day.
Celluloid
Gelatin films had the nice advantage of not exploding, but we photographers apparently just can’t leave exploding stuff alone. Especially nitrocellulose; there’s just something about guncotton that attracts us. Way back in 1855 Alexander Parkes discovered that if you could dissolve your guncotton in camphor (instead of ether and alcohol) you got a thick gel that you could mold into almost any shape, which would then dry to a strong solid. By some definitions, this was the first plastic.
Being a modest man, Parkes called the substance Parkesine and started a company to manufacture and market it. Probably because of the awful name, he went bankrupt. He gave his patents to a friend, Daniel Spill, and apparently also sold them to John Wesley Hyatt, who had also independently developed and patented a similar substance. Both men went on to successfully market the same product as Xylonite and Celluloid, respectively. They were both successful, made lots of money, and entertained themselves by suing each other over patent rights for the next decade.
Hyatt’s first use of celluloid was the manufacture of billiard balls. (I know what you’re thinking, but really this does have some relation to photography. Hang on for a minute.) Until that time billiard balls were made of ivory which made them rather expensive (this was way before PETA and the EPA, so expense was considered the only drawback to ivory billiard balls). Celluloid billiard balls were wildly successful and billiards moved from the parlors of rich men to taverns and sleazy corner pool halls where we enjoy it today. Celluloid billiard balls did have a downside, however. Celluloid is still largely made from nitrocellulose (guncotton), so if you hit the balls a bit too hard...
The photographic world also saw the potential in celluloid and both George Eastman’s Kodak Company and a gentleman named Hannibal Goodwin patented methods for making photographic film on flexible sheets of celluloid. By 1889 Eastman had developed commercial roll film and along with Thomas Edison’s invention of the Kinetoscope in 1891 and Louis Lumiere’s cinématographe in 1895, motion pictures were born. Mr. Goodwin, unfortunately, did not get the patent for his invention until 1898. He started his own film company, but died before he began production. His heirs did quite well, however, suing the Kodak company for patent infringement and receiving the amazing sum of $5,000,000 in 1914. Of course, by that time, writing a check for $5,000,000 was petty cash for Kodak.
Unfortunately, however, history does tend to repeat itself. Once again, photographers were playing with stuff made from nitrocellulose, and once again, bad things would happen. In 1897 the celluloid film being shown in a Paris movie theatre caught fire, burning the theatre to the ground and killing 120 patrons. This caused some countries to require that movie theaters line projection booths with tin, and lock the projectionist inside so if the film burst into flames, there was no chance of the fire spreading. I assume this is when projectionists started getting paid more than the kids selling popcorn.
Safe Film and the End of Nitrocellulose
Despite its risk, celluloid film had so many advantages that it was the main type of photographic and motion picture film used until the 1930s. A different--but similar--chemical, cellulose acetate had been developed in 1904 and the Kodak company actually bought patents and manufactured film from it as early as 1908. It was more expensive than celluloid film and a bit more difficult to work with, so it was not particularly successful. It became easier to manufacture and less expensive in the 1920s. Marketed as ‘safety film’, because it would melt, but not burn, when exposed to fire, cellulose acetate had largely replaced flammable celluloid by the 1930s. The ‘celluloid’ name remained in common use for decades, however.
Photography had done a lot to keep dangerous nitrocellulose in common use, so its probably fitting that photography helped end its use (other than for college pranks and some purposeful explosions). In the early 1900s, a Belgian chemist named Leo Baekeland emigrated to the United States, hoping to make his fortune from chemical inventions. He was unsuccessful for some years, but in 1893 he invented a new type of photographic paper that eliminated the washing and heating steps needed to develop prints. He was nearly bankrupt by this time and approached George Eastman, asking $50,000 for the patents (but having told friends he would happily take $25,000). Eastman immediately offered the amazing sum of $750,000 for Baekeland’s patents.
Baekeland, being no fool, took the money and bought himself a first class chemistry laboratory (and a house and probably some stuff for his wife). In 1907 he produced the first real plastic, which he called Bakelite. Within a few years, Bakelite had a thousand uses: it was an artificial shellac, the major insulator in all electrical appliances, made up most non-metallic car parts, handles for kitchen equipment, plates and dishes, even knobs and telephones. It never exploded and really didn’t even burn easily. Bakelite was THE plastic for a generation. Today it has largely been replaced by newer plastics, but it’s still used as electrical insulator. And it has one other use that continues to make it popular to this day: Bakelite is the substance used to make non-exploding billiard balls.
(Roger Cicala is the founder of LensRentals.com. Visit LensRentals.com to check out that cool lens you've been hankering for, and for some of the best customer service on the Internet! Film canisters image courtesy of flattop341 on Flickr used under an Attribution 2.0 Generic license)